Kappa Opioid Receptor Antagonism in Anhedonia Treatment
Several studies have investigated the efficacy of Kappa Opioid Receptor (KOR) antagonism in treating anhedonia, a core symptom of major depressive disorder (MDD).

Extended Data
This CONSORT diagram shows the flow of participants through a small "fast-fail" proof-of-mechanism trial testing a κ‐opioid antagonist (JNJ 67953964) versus placebo in people with anhedonia.
Primary Data
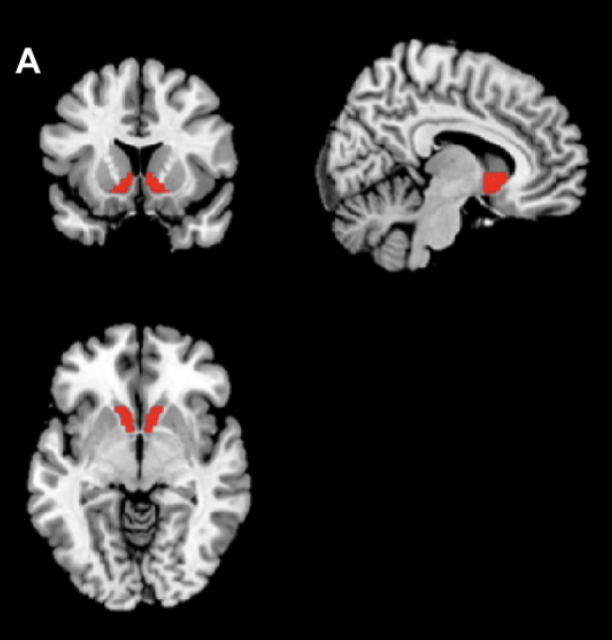
Location of the ventral striatal region of interest (ROI) used in the analysis (panel A), and mean fMRI signal intensity in that ROI during reward anticipation measured at baseline and post‐treatment (panel B) for participants receiving JNJ‐67953964 or placebo. Error bars represent 95% confidence intervals.

FIBSER Side Effect Score Trajectories Over 8 Weeks
The graph tracks mean scores for medication side effects across three dimensions: frequency (solid line), intensity (dotted line), and overall burden (dashed line). All measures showed significant increases from Day 4, peaking at Week 2, followed by a gradual decline through Week 8. The most pronounced changes were seen in frequency and intensity scores, while burden scores remained relatively lower throughout the study period. Error bars represent 95% confidence intervals of the mean. Detailed statistical analyses are available in Supplementary Table 6.
A randomized proof-of-mechanism trial applying the 'fast-fail' approach to evaluating κ-opioid antagonism as a treatment for anhedonia.
Key Insights:
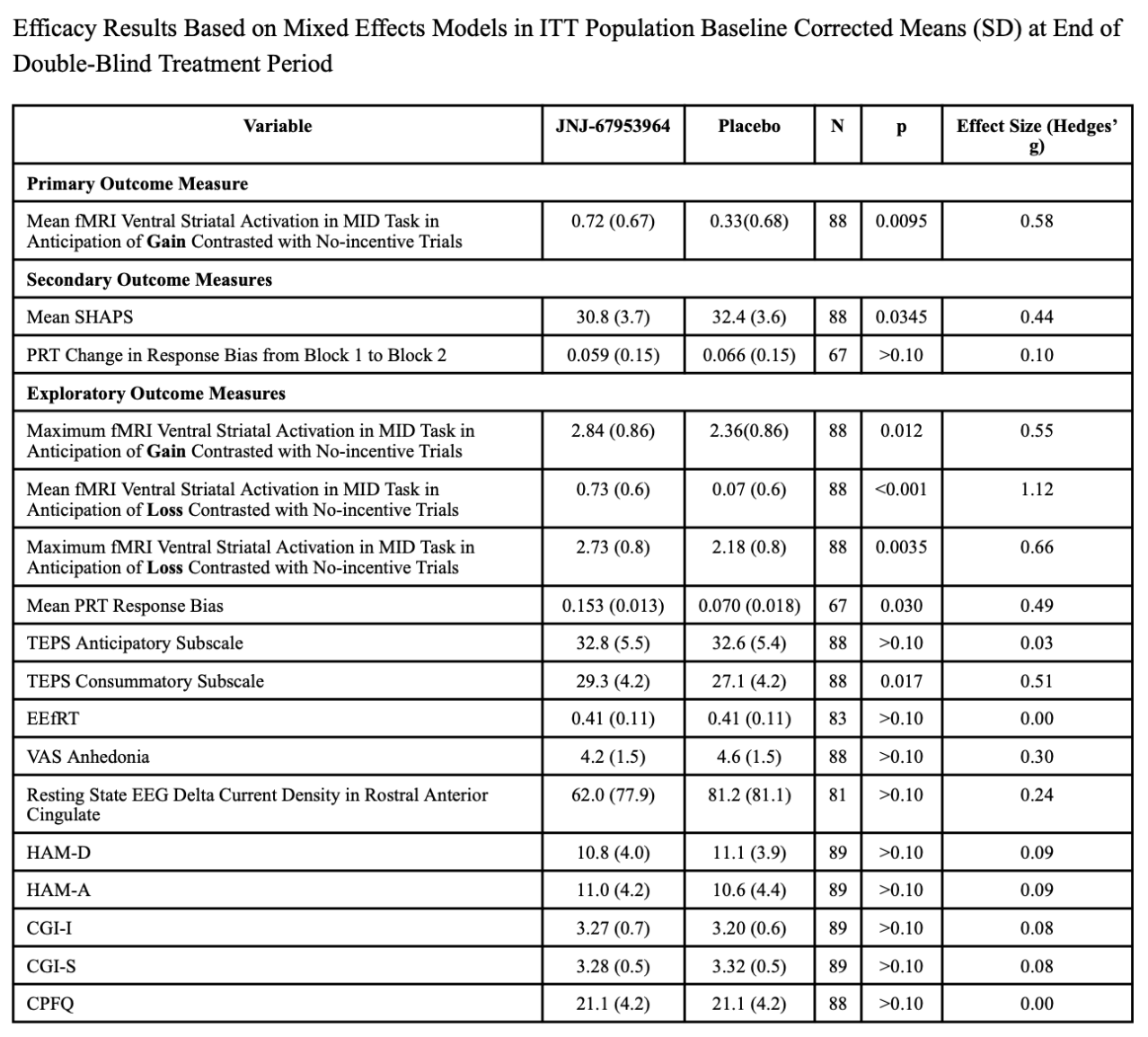
- The primary outcome—mean fMRI ventral striatal activation during reward anticipation—was significantly higher in the JNJ‐67953964 group compared with placebo (p=0.0095), with a moderate effect size (Hedges' g=0.58).
- Clinical outcome–The SHAPS (an anhedonia scale) score was also significantly better in the treatment group (p=0.0345), suggesting a potential clinical benefit on subjective pleasure or hedonic tone. The effect size (g=0.44) indicates a moderate magnitude of difference.
- fMRI outcome–maximum ventral striatal activation in anticipation of gain (p=0.012, g=0.55) and in anticipation of loss (p<0.001, g=1.12)—showed statistically significant differences favoring JNJ‐67953964 (i.e. the investigational drug tested to treat anhedonia).
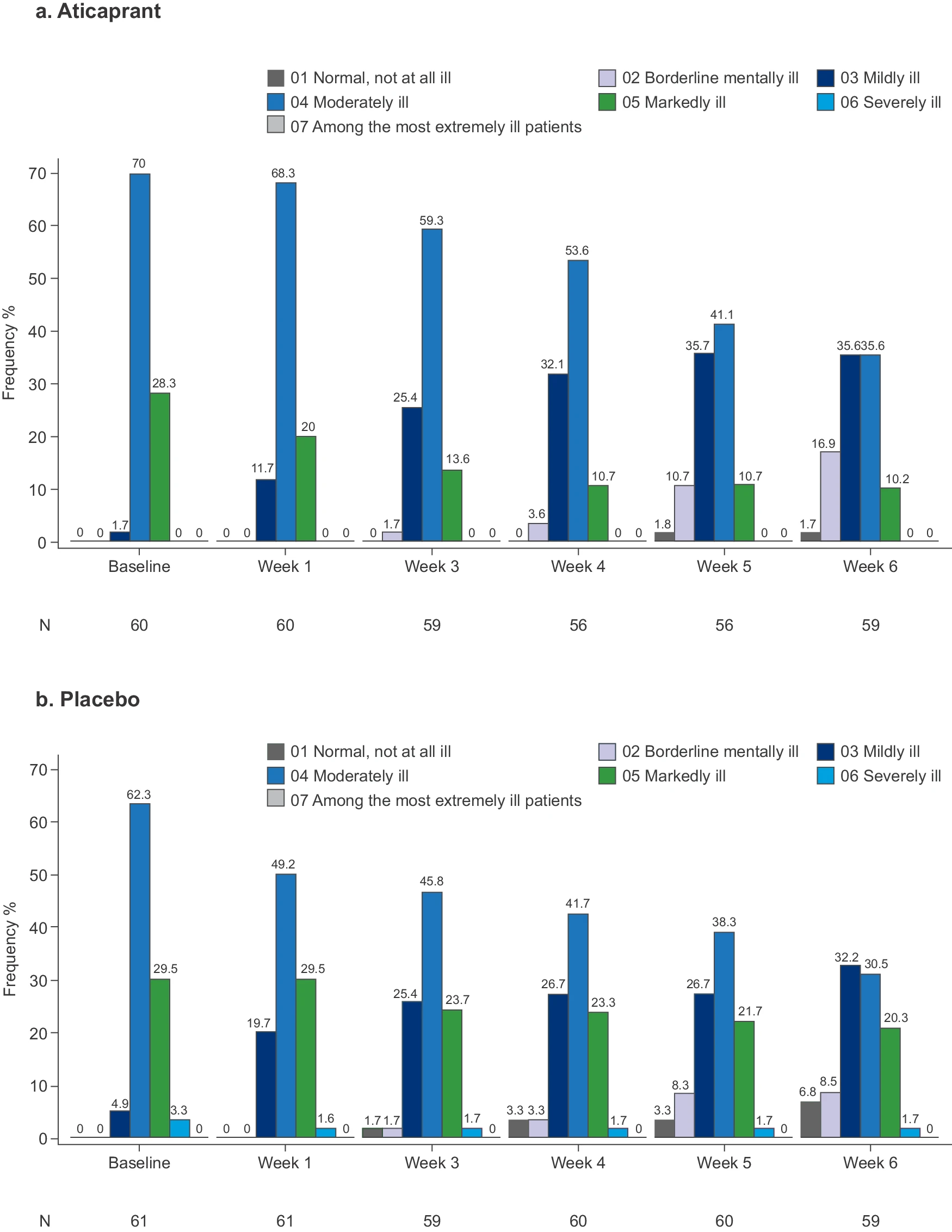
Efficacy and safety of aticaprant, a kappa receptor antagonist, adjunctive to oral SSRI/SNRI antidepressant in major depressive disorder: results of a phase 2 randomized, double-blind, placebo-controlled study
Key Results:
- In the enriched intent-to-treat (eITT) analysis, which included 121 placebo lead-in non-responders, aticaprant showed significant improvement over placebo: The least squares mean difference in MADRS total score at week 6 was -2.1 points (upper limit 1-sided 80% CI: -1.09, 1-sided p = 0.044)4. The effect size was 0.23.
- In the full intent-to-treat (fITT) analysis, which included 166 participants, the results were even more pronounced: The least squares mean difference in MADRS total score at week 6 was -3.1 points (upper limit 1-sided 80% CI: -2.21, 1-sided p = 0.002)4. The effect size increased to 0.36.
- The study found that the treatment effect was more substantial in participants with higher baseline anhedonia, as measured by the Snaith–Hamilton Pleasure Scale (SHAPS).
Computational Modeling Insights
Key Findings:
- KOR Antagonism Shows Two Distinct Effects: Increases policy complexity (cognitive resources) Improves efficiency (better use of resources) These effects are independent, making KOR antagonism potentially useful for multiple conditions.
- Anhedonia's Core Mechanism is More Specific Than Previously Thought: Only linked to reduced efficiency Not associated with reduced policy complexity Challenges traditional views that anhedonia is primarily about reward sensitivity.
- Treatment & Clinical Implications: KOR antagonism could treat both anhedonia (through efficiency improvement) and other cognitive deficits (through complexity increase) Particularly promising for conditions like schizophrenia where cognitive deficits are a major concern Works through dopamine mechanisms in a more nuanced way than previously understood.